A) \[{{H}_{2}}O\]is linear and \[Be{{F}_{2}}\] is angular
B) \[{{H}_{2}}O\]is angular and \[Be{{F}_{2}}\] is linear
C) The electornegativity of F is greater than that of O
D) \[{{H}_{2}}O\]involves hydrogen bonding whereas \[Be{{F}_{2}}\]is a discrete molecule
Correct Answer: B
Solution :
The overall value of the dipole moment of a polar molecule depends on its geometry and shape i.e., vectorial addition of dipole moment of the constituent bonds water has angular structure with bond angle 105° as it has dipole moment. However \[Be{{F}_{2}}\] is a linear molecule since dipole moment summation of all the bonds present in the molecule cancel each other.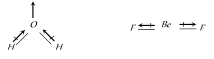
You need to login to perform this action.
You will be redirected in
3 sec
