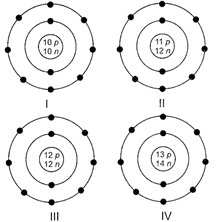
A) I
B) II
C) III
D) IV
Correct Answer: D
Solution :
\[_{13}^{27}Al\] has atomic number 13 and mass number 27. Atomic number = Number of protons =13 and Mass number = Number of protons + number of neutrons Number of neutrons = 27 - 13 = 14 \[\text{A}{{\text{l}}^{\text{3-}}}\] has 10 electrons. Hence, structure IV represents \[\text{A}{{\text{l}}^{\text{3-}}}\]ion.You need to login to perform this action.
You will be redirected in
3 sec
