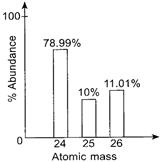
| Study the given mass spectrum of magnesium carefully. |
|
| The number of protons in \[^{\text{26}}\text{Mg}\], number of neutrons in \[^{\text{25}}\text{Mg}\], and the relative atomic mass of Mg are respectively |
A) 12, 13 and 25
B) 16, 12 and 24
C) 14, 13 and 25
D) 12, 13 and 24.32
Correct Answer: D
Solution :
No. of protons in \[^{\text{26}}\text{Mg=12}\] No. of neutrons in \[^{\text{25}}\text{Mg=13}\] Relative atomic mass of Mg \[\text{=}\frac{(24\times 78.99)+(25\times 10)+(26\times 11.01)}{100}\] \[=\frac{1895.76+250+286.26}{100}=24.32\]You need to login to perform this action.
You will be redirected in
3 sec
