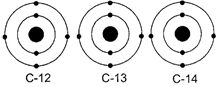
A) C -12 : 6p + 6n, C - 13 : 7p + 6n, C - 14 : 8p + 6n
B) C - 12 : 6p + 6n, C -13 : 6p + 7n, C - 14 : 6p + 8n
C) C - 12 : 6p + 6n, C - 13 : 5p + 8n, C - 14 : 7p + 7n
D) C - 12 : 6p + 6n, C - 13 : 12p +1n, C - 14 : 5p + 9n
Correct Answer: B
Solution :
Isotopes are the atoms of the same element, having same atomic number but different mass numbers. For a neutral atom, Atomic number = Number of protons = Number of electrons Mass number = Number of protons + Number of neutrons. As the mass number of isotopes are 12, 13 and 14 so, number of neutrons will be \[12-6=6,\text{ }13-6=7\] and \[14-6=8\] in C-12, C-13 and C-14 respectively.You need to login to perform this action.
You will be redirected in
3 sec
