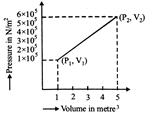
A) \[7.5\times {{10}^{5}}joule~\]
B) \[7.5\times {{10}^{5}}erg\]
C) \[12\times {{10}^{5}}joule\]
D) \[6\times {{10}^{5}}joule\]
Correct Answer: C
Solution :
[c] \[~W={{W}_{1}}+{{W}_{2}}\] \[-{{p}_{0}}(\gamma -1)+\alpha (\gamma -1)\,V={{p}_{0}}-2\alpha V\] \[=12\times {{10}^{5}}\text{joule}\]
You need to login to perform this action.
You will be redirected in
3 sec
