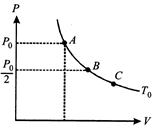
A) \[{{P}_{0}}/2\]
B) \[{{P}_{0}}/4\]
C) \[{{P}_{0}}/6\]
D) \[{{P}_{0}}/8\]
Correct Answer: D
Solution :
[d] Work done by gas in going isothermally from stated to 5 is \[\Delta {{W}_{AB}}=nRT\text{ In}\frac{{{P}_{A}}}{{{P}_{B}}}=nRT\text{ In 2}\] ....(i) Work done by gas in going isothermally from state B to C is \[\Delta {{W}_{BC}}=nRT\text{ In}\frac{{{P}_{B}}}{{{P}_{C}}}=nRT\frac{{{P}_{0}}}{2{{P}_{C}}}\] ...(ii) It is given that \[\Delta {{W}_{BC}}=2\Delta {{W}_{AB}}\] \[\text{In }\frac{{{P}_{0}}}{2{{P}_{C}}}=\text{In}{{\left( \text{2} \right)}^{2}}\text{ }\therefore {{\text{P}}_{C}}=\frac{{{P}_{0}}}{8}\]You need to login to perform this action.
You will be redirected in
3 sec
