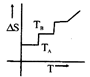
A)
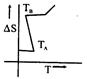
B)
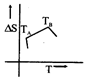
C)
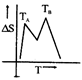
D)
Correct Answer: A
Solution :
[a] For a pure substance \[{{T}_{A}}\] and \[{{T}_{B}}\] represent the same temperature. Hence A is a correct choice.You need to login to perform this action.
You will be redirected in
3 sec
