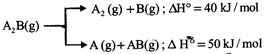 If the molar ratio of \[{{A}_{2}}(g)\] to A (g) is 5 : 3 in a set of product gases, then the energy involved in the decomposition of 1 mole of \[{{A}_{2}}B(g)\] is:
If the molar ratio of \[{{A}_{2}}(g)\] to A (g) is 5 : 3 in a set of product gases, then the energy involved in the decomposition of 1 mole of \[{{A}_{2}}B(g)\] is:
A) \[48.75kJ/mol\]
B) \[43.73\text{ }kJ/mol\]
C) \[46.25\text{ }kJ/mol\]
D) None of these
Correct Answer: B
Solution :
[b] \[{{\Delta }_{r}}H=\frac{5}{8}\times 40+\frac{3}{8}\times 50=43.75kJ/mol\]You need to login to perform this action.
You will be redirected in
3 sec
