A) 14 cal
B) 6 cal
C) 16 cal
D) 66 cal
Correct Answer: B
Solution :
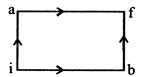
[b] For path iaf, \[Q=50cal~~W=20cal\] By first law of thermodynamics, \[\Delta U=Q-W\text{ }=50-20=30cal.\] For path ibf \[Q'=36cal\] \[W=?\] or, \[W=Q'-\Delta U'\] Since, the change in internal energy does not depend on the path, therefore \[\Delta U'=30cal\] \[\therefore W=Q'-\Delta U'=36-30=6cal.\]
or, \[W=Q'-\Delta U'\] Since, the change in internal energy does not depend on the path, therefore \[\Delta U'=30cal\] \[\therefore W=Q'-\Delta U'=36-30=6cal.\]
You need to login to perform this action.
You will be redirected in
3 sec
