A) isothermal curve slope = adiabatic curve slope
B) isothermal curve slope =\[\gamma \]\[\times \]adiabatic curve slope
C) adiabatic curve slope =\[\gamma \]\[\times \]isothermal curve slope
D) adiabatic curve slope =\[\frac{1}{2}\]\[\times \]isothermal curve slope
Correct Answer: C
Solution :
[c] \[\frac{\text{Slope of adiabatic curve}}{\text{Slope of isothermal curve}}\] \[\text{=}\frac{{{\left( dP/dV \right)}_{adi}}}{{{\left( dP/dV \right)}_{iso}}}=+\gamma \]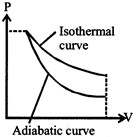 So slop to adiabatic curve is \[\gamma \left( =\frac{{{C}_{P}}}{{{C}_{V}}} \right)\] times of isothermal curve, as clear also from figure.
So slop to adiabatic curve is \[\gamma \left( =\frac{{{C}_{P}}}{{{C}_{V}}} \right)\] times of isothermal curve, as clear also from figure.
You need to login to perform this action.
You will be redirected in
3 sec
