| \[{{H}_{2}}O\left( 1,323\text{ }K \right)\xrightarrow{{}}{{H}_{2}}O\left( g,423K \right)\] |
| Given: \[{{C}_{v,m}}({{H}_{2}}O,I)=75.0J{{K}^{-1}}mo{{l}^{-1}}\]; |
| \[{{C}_{p.m}}({{H}_{2}}O,g)=33.314J{{K}^{-1}}mo{{l}^{-1}}\] |
| \[\Delta {{H}_{vap}}\,\text{at}\,373\,K=40.7\,kJ\text{/}mol\] |
A) \[42.91\text{ }kJ/mol\]
B) \[43086\text{ }kJ/mol\]
C) \[42.6\text{ }kJ/mol\]
D) \[49.6\text{ }kJ/mol\]
Correct Answer: C
Solution :
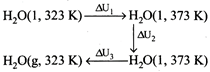 \[{{C}_{v,m}}({{H}_{2}}O,g)=33.314-8.314\] \[=25J/K\text{ }mol\] \[\Delta {{U}_{2}}=\Delta {{H}_{2}}-\Delta {{n}_{g}}RT=37.6\] \[\Delta {{U}_{total}}=\Delta {{u}_{1}}+\Delta {{u}_{2}}+\Delta {{u}_{3}}\] \[={{C}_{v,m}}(l)\Delta T+\Delta {{V}_{vap}}+{{C}_{v,m}}(g)\Delta T\] \[=\frac{75\times 50}{1000}+37.6+\frac{25\times 50}{1000}\] \[=42.6\text{ }kJ/mol\]
\[{{C}_{v,m}}({{H}_{2}}O,g)=33.314-8.314\] \[=25J/K\text{ }mol\] \[\Delta {{U}_{2}}=\Delta {{H}_{2}}-\Delta {{n}_{g}}RT=37.6\] \[\Delta {{U}_{total}}=\Delta {{u}_{1}}+\Delta {{u}_{2}}+\Delta {{u}_{3}}\] \[={{C}_{v,m}}(l)\Delta T+\Delta {{V}_{vap}}+{{C}_{v,m}}(g)\Delta T\] \[=\frac{75\times 50}{1000}+37.6+\frac{25\times 50}{1000}\] \[=42.6\text{ }kJ/mol\]
You need to login to perform this action.
You will be redirected in
3 sec
