A) \[500cal, 50{}^\circ C\]
B) \[1000cal, 100{}^\circ C\]
C) \[1500cal, 200{}^\circ C\]
D) \[1000cal, 200{}^\circ C\]
Correct Answer: C
Solution :
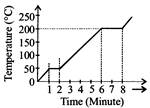
[c] Since specific heat \[=0.6 kcal/g x {}^\circ C=0.6\]\[\operatorname{cal}/gx{}^\circ C\] From graph it is clear that in a minute, the temperature is raised from \[0{}^\circ C\]to \[50{}^\circ C\]. \[\Rightarrow \]Heat required for a minute \[=50\times 0.6\times 50\] =1500 cal. Also from graph. Boiling point of wax is\[200{}^\circ C\].You need to login to perform this action.
You will be redirected in
3 sec
