A) Two \[p\pi -d\pi \] bonds
B) One \[p\pi -d\pi \] bonds
C) Four \[p\pi -d\pi \] bonds
D) Three \[p\pi -d\pi \] bonds
Correct Answer: C
Solution :
[c] Xenon undergo \[s{{p}^{3}}\] hybridization.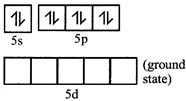
 In the fourth excited state xenon atom, has 8 unpaired electrons
In the fourth excited state xenon atom, has 8 unpaired electrons You need to login to perform this action.
You will be redirected in
3 sec
