A) 0.56K
B) 1.12K
C) \[-0.56K\]
D) \[-1.12K\]
Correct Answer: B
Solution :
[b] As \[\Delta {{T}_{f}}={{K}_{f}}.m\] For,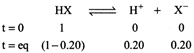 Total no. of moles \[=1-0.20+0.20+0.20\] \[=1+0.20=1.2\] \[\therefore \text{ }\Delta {{T}_{f}}=1.2\times 1.86\times 0.5=1.1160\approx 1.12K\]
Total no. of moles \[=1-0.20+0.20+0.20\] \[=1+0.20=1.2\] \[\therefore \text{ }\Delta {{T}_{f}}=1.2\times 1.86\times 0.5=1.1160\approx 1.12K\]
You need to login to perform this action.
You will be redirected in
3 sec
