A) Partial ionization
B) Dissociation
C) Complex formation
D) Association
Correct Answer: D
Solution :
[d] Acetic acid contain carboxylic group - COOH which can form H-bonding so acetic acid dimerises.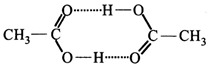
You need to login to perform this action.
You will be redirected in
3 sec
