A) 274.339K
B) \[-1.339K\]
C) 257.3 K
D) \[-1.339{}^\circ C\]
Correct Answer: D
Solution :
[d] \[\Delta {{T}_{f}}=i{{K}_{f}}m\]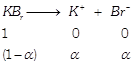 \[i=1+\alpha \] Here \[\alpha =0.8\] (80% ionization) =1.8 \[\Delta {{T}_{f}}=\left( 1.8 \right)\left( 1.86 \right)\left( 0.4 \right)=1.339\] \[{{T}_{f}}=-1.339{}^\circ C\]
\[i=1+\alpha \] Here \[\alpha =0.8\] (80% ionization) =1.8 \[\Delta {{T}_{f}}=\left( 1.8 \right)\left( 1.86 \right)\left( 0.4 \right)=1.339\] \[{{T}_{f}}=-1.339{}^\circ C\]
You need to login to perform this action.
You will be redirected in
3 sec
