A) 0.16
B) 0.05
C) 0.1
D) 0.2
Correct Answer: A
Solution :
[a] (i) \[i=\frac{No.\text{ }of\text{ }particles\text{ }after\text{ }ionisation}{No.\text{ }of\text{ }particles\text{ }before\text{ }ionisation}\] (ii) \[\Delta {{T}_{b}}=i\times {{K}_{b}}\times m\]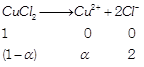 \[i=\frac{1+2\alpha }{1},=1+2\alpha \] Assuming 100% ionization So, \[i=1+2=3\] \[\Delta {{T}_{b}}=3\times 0.52\times 0.1=0.156\approx 0.16\] \[\left( m=\frac{13.44}{134.4}=0.1 \right)\]
\[i=\frac{1+2\alpha }{1},=1+2\alpha \] Assuming 100% ionization So, \[i=1+2=3\] \[\Delta {{T}_{b}}=3\times 0.52\times 0.1=0.156\approx 0.16\] \[\left( m=\frac{13.44}{134.4}=0.1 \right)\]
You need to login to perform this action.
You will be redirected in
3 sec
