A) \[s{{p}^{3}}\], two
B) \[ds{{p}^{2}}\], zero
C) \[ds{{p}^{2}}\], one
D) \[s{{p}^{3}}\], zero
Correct Answer: A
Solution :
[a] \[{{[Ni{{L}_{4}}]}^{2-}}\]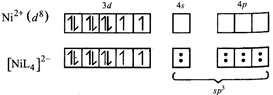 i.e, no. of unpaired electron = 2 hybridization -\[s{{p}^{3}}\].
i.e, no. of unpaired electron = 2 hybridization -\[s{{p}^{3}}\].
You need to login to perform this action.
You will be redirected in
3 sec
