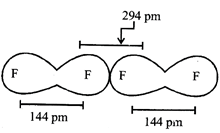
A) 219 pm, 72 pm
B) 75 pm, 72 pm
C) 147 pm, 72 pm
D) 147 pm, 144 pm
Correct Answer: C
Solution :
[c] Covalent radius is radius of an atom in its bound state i.e., in fluorine it is half of distance between two covalently bonded fluorine atoms; van der Waal radii is one- half of the distance between the nuclei of two identical non-bonded isolated atoms. These atoms are attracted toward each other through weak van der Waal's force hence van der Waal radii are very large.You need to login to perform this action.
You will be redirected in
3 sec
