A) stronger \[\sigma \] bond between B and F in\[~B{{F}_{3}}\] as compared to that between C and F in \[C{{F}_{4}}\].
B) significant \[p\pi -p\pi \] interaction between B and F in\[B{{F}_{3}}\] whereas there is no possibility of such interaction between C and F in\[C{{F}_{4}}\].
C) lower degree of \[p\pi -p\pi \] interaction between B and F in \[~B{{F}_{3}}\] than that between C and F in \[C{{F}_{4}}\]
D) Smaller size of \[B-atom\] as compared to that of C-atom.
Correct Answer: B
Solution :
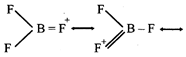
[b] The delocalised \[p\pi -p\pi \] bonding between filled p-orbital of F and vacant p-orbital of B leads to shortening of \[B-F\] bond length which results in higher bond dissociation energy of the \[B-F\]bond.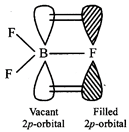

You need to login to perform this action.
You will be redirected in
3 sec
