A) \[ds{{p}^{2}}\] hybridization
B) \[s{{p}^{3}}d\] hybridization
C) \[ds{{p}^{3}}\] hybridization
D) \[s{{p}^{3}}{{d}^{2}}\] hybridization
Correct Answer: D
Solution :
[d]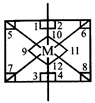 \[s{{p}^{3}}{{d}^{2}}\] hybridisation Number of \[90{}^\circ \] angle between bonds = 12
\[s{{p}^{3}}{{d}^{2}}\] hybridisation Number of \[90{}^\circ \] angle between bonds = 12
You need to login to perform this action.
You will be redirected in
3 sec
