A) \[2\times {{10}^{-10}}esu\]
B) \[4.28\times {{10}^{-10}}esu\]
C) \[3.22\times {{10}^{-10}}esu\]
D) \[1.602\times {{10}^{-19}}C\]
Correct Answer: C
Solution :
[c] \[\mu =1.85\,D=1.85\times {{10}^{-18}}esu\,cm=q\times d\]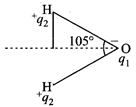
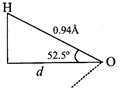 \[cos\text{ }52.5{}^\circ =\frac{d}{0.94\overset{{}^\circ }{\mathop{A}}\,}\Rightarrow d=0.609\times 0.94\overset{{}^\circ }{\mathop{A}}\,\] \[=0.572\overset{{}^\circ }{\mathop{A}}\,\] \[\therefore \,\mu =q\times d\] \[{{q}_{1}}=\frac{\mu }{d}=\frac{1.85D}{0.572\overset{{}^\circ }{\mathop{A}}\,}\] \[=\frac{1.85\times {{10}^{-18}}esu\,cm}{0.572\times {{10}^{-8}}cm}\] \[=3.2\times {{10}^{-10}}esu\]
\[cos\text{ }52.5{}^\circ =\frac{d}{0.94\overset{{}^\circ }{\mathop{A}}\,}\Rightarrow d=0.609\times 0.94\overset{{}^\circ }{\mathop{A}}\,\] \[=0.572\overset{{}^\circ }{\mathop{A}}\,\] \[\therefore \,\mu =q\times d\] \[{{q}_{1}}=\frac{\mu }{d}=\frac{1.85D}{0.572\overset{{}^\circ }{\mathop{A}}\,}\] \[=\frac{1.85\times {{10}^{-18}}esu\,cm}{0.572\times {{10}^{-8}}cm}\] \[=3.2\times {{10}^{-10}}esu\]
You need to login to perform this action.
You will be redirected in
3 sec
