A) \[Xe{{F}_{4}}\]
B) \[I{{F}_{5}}\]
C) \[Sb{{F}_{5}}\]
D) \[C{{F}_{4}}\]
Correct Answer: B
Solution :
[b] The geometry of \[I{{F}_{5}}\] is square Pyramidal with an unsymmetric charge distribution therefore this molecule is polar.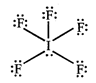
You need to login to perform this action.
You will be redirected in
3 sec
