A)
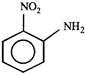
B)
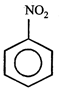
C)
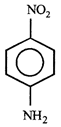
D)
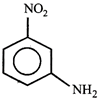
Correct Answer: C
Solution :
[c]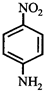 Dipole moment = (Distance between opposite charges) \[\times \](charge, q) \[\mu =q\times d\] So, greater the distance between the opposite charges higher the dipole. Due to the resonance the greater charge separation occurs between charges due to linearity
Dipole moment = (Distance between opposite charges) \[\times \](charge, q) \[\mu =q\times d\] So, greater the distance between the opposite charges higher the dipole. Due to the resonance the greater charge separation occurs between charges due to linearity 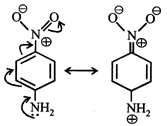
You need to login to perform this action.
You will be redirected in
3 sec
