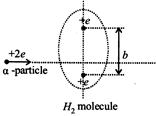
A) \[\frac{b}{2}\]
B) \[\frac{b}{2\sqrt{2}}\]
C) \[\frac{b}{\sqrt{2}}\]
D) None of these
Correct Answer: A
Solution :
[a] \[\frac{1}{{{\lambda }_{\alpha }}}=\frac{3R}{4}{{\left( Z-1 \right)}^{2}}\] \[\Rightarrow \left( Z-1 \right)=\sqrt{\frac{4}{3R{{\lambda }_{\alpha }}}}=\sqrt{\frac{4}{3\times 1.1\times {{10}^{7}}\times 1.8\times {{10}^{-10}}}}\]\[=\frac{200}{3}\sqrt{\frac{5}{33}}=\frac{78}{3}=26\] \[\Rightarrow Z=26+1=27\]You need to login to perform this action.
You will be redirected in
3 sec
