A) \[1.05\times {{10}^{-34}}J-s\]
B)
\[3.16\times {{10}^{-34}}J-s\] [b] \[{{E}_{p}}=\frac{2{{K}_{ex}}}{{{\left( {{x}^{2}}+\frac{{{b}^{2}}}{4} \right)}^{3/2}}}\] For maximum \[{{E}_{p}}\] \[\frac{d{{E}_{p}}}{dx}=0\] \[\Rightarrow x=\frac{b}{2\sqrt{2}}\] 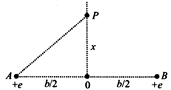
C) \[2.11\times {{10}^{-34}}J-s\]
D) \[4.22\times {{10}^{-34}}J-s\]
Correct Answer: A
Solution :
[a] Electron after absorbing 10.2 eV energy goes to its first excited state (n = 2) from ground state (n = 1). \[\therefore \] Increase in momentum \[=\frac{h}{2\pi }\] \[=\frac{6.6\times {{10}^{-34}}}{6.28}=1.05\times {{10}^{-34}}J-s\]You need to login to perform this action.
You will be redirected in
3 sec
