A) different type of hybridisation in the two amines,
B) protonated dimethyl amine is more solvated than methyl amine.
C) protonated dimethyl amine is more solvated than the protonated methyl amine.
D) protonated dimethyl amine is less stable than the protonated methyl amine.
Correct Answer: D
Solution :
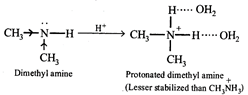
[d] The basic character of an amine in water is determined by (i) electron availability on the N atom and (ii) the extent of stabilization of the cation (protonated amine) due to solvation by hydrogen bonding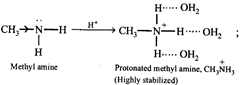
You need to login to perform this action.
You will be redirected in
3 sec
