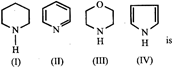
A) IV>I>III>II
B) I>III>II>IV
C) III>I>IV>II
D) II>I>III>IV
Correct Answer: B
Solution :
[b] The order of basicity is I > III > II > IV. The lone pair of electrons on N is more readily available for protonation in I and III then in II. Ill contains an oxygen atom which has - I effect due to which it is less basic than I. In compound IV lone pair of \[{{e}^{-}}s\] on N-atom is contributed towards the aromatic sextet formation and hence is not at all available for protonation. Hence option [b] is correct.You need to login to perform this action.
You will be redirected in
3 sec
