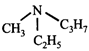
A) it is a chiral molecule
B) It exists in two resolvable optically active forms
C) Both [a] and [b]
D) Neither [a] nor [b]
Correct Answer: A
Solution :
[a] In amines, N is \[s{{p}^{3}}\] hybridised and thus has pyramidal shape. In the given structure, since the three alkyl groups are different, and the fourth corner of the pyramid is occupied by lone pair of electrons, the molecule is chiral. However, the two enantiomers of the amine are not resolvable because of their rapid interconversion through a transition state having planar structure (\[s{{p}^{2}}\]hybridised nitrogen)You need to login to perform this action.
You will be redirected in
3 sec
