A)
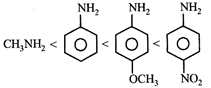
B)

C)
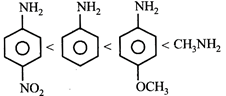
D)
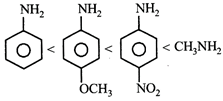
Correct Answer: C
Solution :
[c] Aliphatic amines are more basic than aromatic amines thus methylamine is most basic. Electron donating groups increase the basicity whereas electron withdrawing groups decrease the basicity of the aromatic amines. Thus p-methoxyaniline is more basic then aniline which is further more basic then p-Nitroaniline.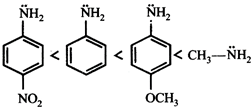
You need to login to perform this action.
You will be redirected in
3 sec
