A)
\[O-H\] bond is more polar than ![]() group
group
B) carboxylate ion gets ionized
C) carboxylate ion gets stabilised by resonance
D) it exists as \[-COOH\] and there is no carbonyl group
Correct Answer: C
Solution :
[c] Due to resonance in carboxylate ion, the double bond character of \[C=O\] bond in carboxylic acids is greatly reduced as compared to that in aldehydes and ketones.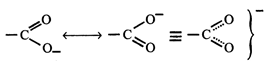
You need to login to perform this action.
You will be redirected in
3 sec
