A) Alcohols are weaker acids than water.
B) Acid strength of alcohols decreases in the following \[RC{{H}_{2}}OH>{{R}_{2}}CHOH>{{R}_{3}}COH.\]
C) Carbon-oxygen bond length in methanol, \[C{{H}_{3}}OH\] is shorter than that of \[C-O\] bond length in phenol.
D)
The bond angle ![]() in methanol is\[108.9{}^\circ \].
in methanol is\[108.9{}^\circ \].
Correct Answer: C
Solution :
[c] The \[C-O\] bond length in alcohols is 142 pm and in Phenol it is 136 pm. The \[C-O\] bond length in phenol is .shorter than methanol it is due to the conjugation of unshared pair of electrons on oxygen with the ring, which imparts double bond character to the \[C-O\] bond.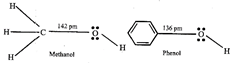
You need to login to perform this action.
You will be redirected in
3 sec
