| Assertion: In a reaction \[Zn(s)+CuS{{O}_{4}}(aq)\to \] \[ZnS{{O}_{4}}(aq)+Cu(s)\], \[Zn\] is a reductant but itself get oxidized. |
| Reason: In a redox reaction, oxidant is reduced by accepting electrons and reductant is oxidized by losing electrons. |
A) If both assertion and reason are true and the reason is the correct explanation of the assertion.
B) If both assertion and reason are true but reason is not the correct explanation of the assertion.
C) If assertion is true but reason is false.
D) If the assertion and reason both are false.
E) If assertion is false but reason is true.
Correct Answer: A
Solution :
Both assertion and reason are true and reason is the correct explanation of assertion.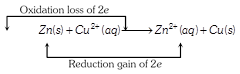
You need to login to perform this action.
You will be redirected in
3 sec
