A) 350 cm3
B) 300 cm3
C) 250 cm3
D) 22 cm3
Correct Answer: B
Solution :
According to Boyle's law, pressure and volume are inversely proportional to each other i.e. \[P\propto \frac{1}{V}\]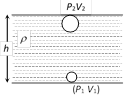 Þ \[{{P}_{1}}{{V}_{1}}={{P}_{2}}{{V}_{2}}\] Þ \[({{P}_{0}}+h{{\rho }_{w}}g){{V}_{1}}={{P}_{0}}{{V}_{2}}\] \[\Rightarrow {{V}_{2}}=\left( 1+\frac{h{{\rho }_{w}}g}{{{P}_{0}}} \right){{V}_{1}}\] Þ \[{{V}_{2}}=\left( 1+\frac{47.6\times {{10}^{2}}\times 1\times 1000}{70\times 13.6\times 1000} \right)\ \,{{V}_{1}}\] \[\Rightarrow {{V}_{2}}=(1+5)50\,c{{m}^{3}}=300\,c{{m}^{3}}.\] [As \[{{P}_{2}}={{P}_{0}}=70\,cm\] of Hg \[=70\times 13.6\times 1000\]]
Þ \[{{P}_{1}}{{V}_{1}}={{P}_{2}}{{V}_{2}}\] Þ \[({{P}_{0}}+h{{\rho }_{w}}g){{V}_{1}}={{P}_{0}}{{V}_{2}}\] \[\Rightarrow {{V}_{2}}=\left( 1+\frac{h{{\rho }_{w}}g}{{{P}_{0}}} \right){{V}_{1}}\] Þ \[{{V}_{2}}=\left( 1+\frac{47.6\times {{10}^{2}}\times 1\times 1000}{70\times 13.6\times 1000} \right)\ \,{{V}_{1}}\] \[\Rightarrow {{V}_{2}}=(1+5)50\,c{{m}^{3}}=300\,c{{m}^{3}}.\] [As \[{{P}_{2}}={{P}_{0}}=70\,cm\] of Hg \[=70\times 13.6\times 1000\]]
You need to login to perform this action.
You will be redirected in
3 sec
