A) Acetone + Chloroform
B) Water + Nitric acid
C) Water + Hydrochloric acid
D) Benzene + Methanol
Correct Answer: D
Solution :
[d] (1) \[C{{H}_{3}}COC{{H}_{3}}\]and \[CHC{{l}_{3}}\]are volatile. On mixing, there is an increase in intermolecular H-bonding due to the formation of chloretone.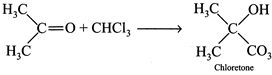 Thus, force of attraction between \[(CHC{{l}_{3}}-C{{H}_{3}}COC{{H}_{3}})\]molecules is larger than between \[(CHC{{l}_{3}}-CHC{{l}_{3}})\]and \[(CHCOC{{H}_{3}}-C{{H}_{3}}COO{{H}_{3}})\]molecules. Thus, vaporization decreases, a case of negative deviation. (2) As in [c], negative deviation. (3) When \[HCl\] is mixed with \[{{H}_{2}}O\], force of attraction between \[HCl\] and \[{{H}_{2}}O\] molecules is increased (due to dissolution), hence vaporization decreased, a case of negative deviation. (4) \[C{{H}_{3}}OH\] molecules are joined by intermolecular H-bonding which provides a liquid state. On adding benzene. H-bonding breaks and the force of attraction between \[C{{H}_{3}}OH\] and benzene molecules is less than between \[C{{H}_{3}}OH-C{{H}_{3}}OH\] and between benzene-benzene. Hence, more is the vaporization of mixture. Thus a case of positive deviation.
Thus, force of attraction between \[(CHC{{l}_{3}}-C{{H}_{3}}COC{{H}_{3}})\]molecules is larger than between \[(CHC{{l}_{3}}-CHC{{l}_{3}})\]and \[(CHCOC{{H}_{3}}-C{{H}_{3}}COO{{H}_{3}})\]molecules. Thus, vaporization decreases, a case of negative deviation. (2) As in [c], negative deviation. (3) When \[HCl\] is mixed with \[{{H}_{2}}O\], force of attraction between \[HCl\] and \[{{H}_{2}}O\] molecules is increased (due to dissolution), hence vaporization decreased, a case of negative deviation. (4) \[C{{H}_{3}}OH\] molecules are joined by intermolecular H-bonding which provides a liquid state. On adding benzene. H-bonding breaks and the force of attraction between \[C{{H}_{3}}OH\] and benzene molecules is less than between \[C{{H}_{3}}OH-C{{H}_{3}}OH\] and between benzene-benzene. Hence, more is the vaporization of mixture. Thus a case of positive deviation.
You need to login to perform this action.
You will be redirected in
3 sec
