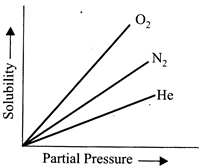
A) \[{{O}_{2}}>{{N}_{2}}>He\]
B) \[{{O}_{2}}<{{N}_{2}}<He\]
C) \[{{O}_{2}}={{N}_{2}}=He\]
D) \[{{O}_{2}}>{{N}_{2}}>He\]
Correct Answer: B
Solution :
[b]| Gas | Temperature | \[{{K}_{H}}\](K bar) |
| \[He\] | 293 | 144.97 |
| \[{{N}_{2}}\] | 293 | 76.48 |
| \[{{O}_{2}}\] | 293 | 34.86 |
You need to login to perform this action.
You will be redirected in
3 sec
