A) 71.46
B) 7.14
C) 85.76
D) 88.14
Correct Answer: D
Solution :
[d] \[\Delta T={{k}_{b}}\cdot m\] \[={{k}_{b}}\cdot \frac{{{W}_{B}}}{{{M}_{{{W}_{B}}}}}\times \frac{1000}{{{W}_{A}}}\]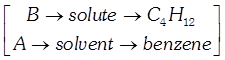 \[=2.57\times \frac{10}{180}\times \frac{1000}{20}\] \[={{7.14}^{0}}C\] Now, \[\Delta T={{T}_{b}}-{{T}_{b}}^{0}\] \[\Rightarrow {{T}_{b}}=\Delta {{T}_{b}}+{{T}_{b}}^{0}\] \[=7.14+81=88.14{}^\circ C\]
\[=2.57\times \frac{10}{180}\times \frac{1000}{20}\] \[={{7.14}^{0}}C\] Now, \[\Delta T={{T}_{b}}-{{T}_{b}}^{0}\] \[\Rightarrow {{T}_{b}}=\Delta {{T}_{b}}+{{T}_{b}}^{0}\] \[=7.14+81=88.14{}^\circ C\]
You need to login to perform this action.
You will be redirected in
3 sec
