A) \[{{H}^{+}},\,{{H}_{2}}O\]
B) \[C{{r}_{2}}{{O}^{2-}}_{7},C{{r}^{3+}}\]
C) \[{{I}^{-}},{{I}_{2}}\]
D) None of them are balanced
Correct Answer: C
Solution :
[c]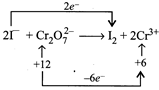 (I) \[C{{r}_{2}}{{O}_{7}}^{2-}+6{{e}^{-}}\to 2C{{r}^{3+}}\] (II) \[2{{I}^{-}}\to {{I}_{2}}+2{{e}^{-}}\] To balance electron (II) is to be multiplied by (3). Thus, \[\underline{6{{I}^{-}}}+C{{r}_{2}}{{O}^{2-}}_{7}+14{{H}^{+}}\to \underline{{{3I}_{2}}}+2C{{r}^{3+}}+7{{H}_{2}}O\] Thus, \[{{I}^{-}}\] and \[{{I}_{2}}\] are not balanced.
(I) \[C{{r}_{2}}{{O}_{7}}^{2-}+6{{e}^{-}}\to 2C{{r}^{3+}}\] (II) \[2{{I}^{-}}\to {{I}_{2}}+2{{e}^{-}}\] To balance electron (II) is to be multiplied by (3). Thus, \[\underline{6{{I}^{-}}}+C{{r}_{2}}{{O}^{2-}}_{7}+14{{H}^{+}}\to \underline{{{3I}_{2}}}+2C{{r}^{3+}}+7{{H}_{2}}O\] Thus, \[{{I}^{-}}\] and \[{{I}_{2}}\] are not balanced.
You need to login to perform this action.
You will be redirected in
3 sec
