A) The nitrogen atom rapidly inverts its configuration leading to a racemic mixture
B) it isomerizes rapidly with the achiral isomer trimethylamine
C) the favoured configuration is not chiral
D) the C-N bond is not stable under conditions used for resolution
Correct Answer: A
Solution :
[a] The N-atom of ethylmethylamine undergoes fast inversion, so it cannot be resolved under normal conditions.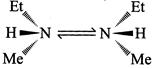
You need to login to perform this action.
You will be redirected in
3 sec
