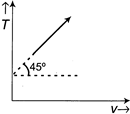
A)
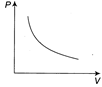
B)
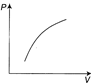
C)
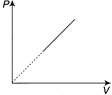
D)
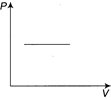
Correct Answer: A
Solution :
[a] Here, \[T=V\tan 45{}^\circ +{{T}_{0}}\Rightarrow T=V+{{T}_{0}}\] And \[P=\frac{nRT}{V},\] \[\therefore \frac{PV}{R}=V+{{T}_{0}}\] (Since, n=1) \[(P-R)V=R{{T}_{0}}\] Therefore, graph will be rectangular hyperbola.You need to login to perform this action.
You will be redirected in
3 sec
