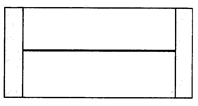
A) \[\frac{3}{8}{{T}_{0}}\]
B) \[\frac{3}{4}{{T}_{0}}\]
C) \[\frac{11}{8}{{T}_{0}}\]
D) \[\frac{13}{8}{{T}_{0}}\]
Correct Answer: C
Solution :
[c] \[T+{{P}_{0}}A=PA\] \[T=(P-{{P}_{0}})A=\frac{3}{8}{{P}_{0}}A\] (given) \[P=\frac{3}{8}{{P}_{0}}+{{P}_{0}}=\frac{11}{8}{{P}_{0}}\] Now volume is constant by string So. \[P\propto T\] Initial temperature is \[{{T}_{0}}.\]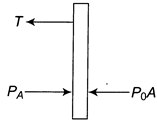
You need to login to perform this action.
You will be redirected in
3 sec
