A) \[\frac{1}{2}M{{V}^{2}}+\frac{3}{2}nRT\]
B) \[\frac{1}{2}M{{V}^{2}}\]
C) \[\frac{1}{2}M{{V}^{2}}+\frac{5}{2}nRT\]
D) \[\frac{5}{2}nRT\]
Correct Answer: C
Solution :
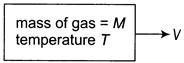
[c] The kinetic energy of gas W.r.t. center of mass of the system K.E.\[=\frac{5}{2}nRT\] Kinetic energy of gas w.r.t ground =kinetic energy of centre of mass w.r.t. ground +Kinetic energy of gas w.r.t. centre of mass. \[K.E.=\frac{1}{2}M{{V}^{2}}+\frac{5}{2}nRT\]You need to login to perform this action.
You will be redirected in
3 sec
