A) \[2.5{{P}_{0}}{{V}_{0}}\]
B) \[1.4{{P}_{0}}{{V}_{0}}\]
C) \[3.9{{P}_{0}}{{V}_{0}}\]
D) \[1.1{{P}_{0}}{{V}_{0}}\]
Correct Answer: C
Solution :
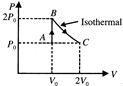
[c] \[{{Q}_{AB}}=\Delta {{U}_{AB}}+{{W}_{AB}}\] \[{{W}_{AB}}\]=0 \[\Delta {{U}_{AB}}=\frac{f}{2}nR\Delta T\] \[\frac{f}{2}(\Delta PV)\Delta {{U}_{AB}}=\frac{5}{2}(\Delta PV)\] \[{{Q}_{AB}}=2.5{{P}_{0}}{{V}_{0}}\] Process BC, \[{{Q}_{BC}}=\Delta {{U}_{BC}}+{{W}_{BC}}=0+2{{P}_{0}}{{V}_{0}}\log 2=1.4{{P}_{0}}{{V}_{0}}\] \[{{Q}_{net}}={{Q}_{AB}}+{{Q}_{BC}}=3.9{{P}_{0}}{{V}_{0}}\]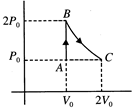
You need to login to perform this action.
You will be redirected in
3 sec
