A) 4200 J
B) 500 J
C) 9000J
D) 9800J
Correct Answer: D
Solution :
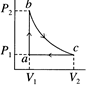
[d] \[{{Q}_{ab}}=+7000=\mu {{C}_{v}}(1000-300)\] ...(i) For the process ca: \[{{T}_{a}}=300K,\]\[{{T}_{c}}={{T}_{b}}=1000K\] \[{{Q}_{Ca}}=\mu {{C}_{P}}(300-1000)=-\mu {{C}_{p}}\times 700\] \[=-\mu ({{C}_{v}}+R)700\] ...(ii) For carbon monoxide: \[{{T}_{a}}=300K,\]\[{{T}_{c}}={{T}_{h}}=1000K\] \[{{(\Delta Q)}_{ca}}=\mu {{C}_{p}}(300-1000)=-\mu {{C}_{P}}\times 700\] \[=-\mu ({{C}_{v}}+R)700\] For carbon monoxide: \[\gamma =\frac{7}{5}\] \[{{C}_{v}}=\frac{R}{\gamma -1}=\frac{R}{\frac{7}{5}-1}=\frac{5R}{2}\] ...(iii) Hence, form Eq. (i) \[7000=\mu \frac{5R}{2}\times 700\]Or \[\mu R=\frac{20}{5}=4\] \[{{Q}_{ca}}=-(7000+4\times 700)=-9800J\] Negative sing shows that heat is ejected.You need to login to perform this action.
You will be redirected in
3 sec
