A)
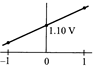
B)
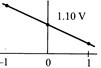
C)
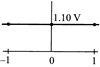
D)
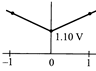
Correct Answer: B
Solution :
[b] When log \[Q=0,E={{E}^{0}}=1.10V\] The value of cell potential \[({{E}_{cell}})\]will decrease with increase in the value of \[{{\log }_{10}}\]Q; as indicated by the Nernst equation\[E={{E}^{o}}-\frac{0.059}{n}{{\log }_{10}}Q\]You need to login to perform this action.
You will be redirected in
3 sec
