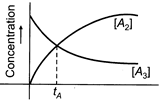
A) 75%
B) 50%
C) 40%
D) 30%
Correct Answer: C
Solution :
[c] \[{{A}_{3}}\to \frac{3}{2}{{A}_{2}}\] \[A-x=\frac{3}{2}x\] \[A-x=\frac{3}{2}x\] or \[x=\frac{2}{5}A=40%\,of\,A.\] % of A.You need to login to perform this action.
You will be redirected in
3 sec
