A) The electronegativity of F is greater than that of 0
B) \[{{H}_{2}}O\] involves hydrogen bonding whereas \[Be{{F}_{2}}\] is a discrete molecule
C) \[{{H}_{2}}O\] is linear and \[Be{{F}_{2}}\] is angular
D) \[{{H}_{2}}O\] is angular and \[Be{{F}_{2}}\] is linear
Correct Answer: D
Solution :
[d] In a linear symmetrical molecule like \[\left[ Be{{F}_{2}} \right]\]the bond angle between three atoms is \[180{}^\circ \]hence the polarity due to one bond is cancelled by the equal polarity due to other bond, while it is not so in the case of angular molecules like \[{{H}_{2}}O\].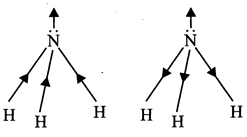
You need to login to perform this action.
You will be redirected in
3 sec
