A) \[S{{F}_{6}}\]
B) \[P{{F}^{\odot }}_{6}\]
C) \[Si{{F}^{2-}}_{6}\]
D) \[Xe{{F}_{6}}\]
Correct Answer: D
Solution :
[d] \[A{{B}_{6}}\] E type (\[:Xe{{F}_{6}}\]) have \[s{{p}^{3}}{{d}^{3}}\]hybridisation with TBP geometry and it should have pentagonal pyramid shape. But this case is an exception in which it has distorted structure.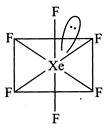
You need to login to perform this action.
You will be redirected in
3 sec
