A) N: tetrahedral, \[s{{p}^{3}}\], B: tetrahedral, \[s{{p}^{3}}\]
B) N: pyramidal, \[s{{p}^{3}}\]', B: pyramidal, \[s{{p}^{3}}\]
C) N: pyramidal, \[s{{p}^{3}}\], B: planar, \[s{{p}^{2}}\]
D) N: pyramidal, \[s{{p}^{3}}\], B: tetrahedral, \[s{{p}^{3}}\]
Correct Answer: A
Solution :
[a] The 1: 1 complex both \[B{{F}_{3}}\]and \[N{{H}_{3}}\]is represented as follows: in this complex both N and B attain tetrahedral geometry and \[s{{p}^{3}}\]hybridization in the coordinately formed structure. During coordinate bond formation hybridized state of donor doesn?t change but hybridized state of acceptor changes.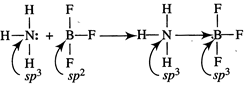
You need to login to perform this action.
You will be redirected in
3 sec
