| Two elements X and Y have following electronic Configurations |
| \[X:1{{s}^{2}}\,2{{s}^{2}}\,2{{p}^{2}}\,3{{s}^{2}}\,3{{p}^{6}}4{{s}^{2}}\] |
| \[Y:1{{s}^{2}}\,2{{s}^{2}}\,2{{p}^{6}}\,3{{s}^{2}}\,3{{p}^{5}}\] |
| The expected compound formed by combination of X and Y will be expressed as |
A) \[X{{Y}_{2}}\]
B) \[{{X}_{5}}{{Y}_{2}}\]
C) \[{{X}_{2}}{{Y}_{5}}\]
D) \[{{X}_{{}}}{{Y}_{5}}\]
Correct Answer: A
Solution :
[a] Valency of element X is 2 (electrons in the outermost shell) while that of element Y is 1 (electron required in the outermost shell to complete octet). So, the formula of the compound between X and Y is \[X{{Y}_{2}}\]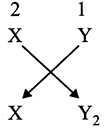
You need to login to perform this action.
You will be redirected in
3 sec
